Niels Bohr
Lived 1885 – 1962.
Niels Bohr completely transformed our view of the atom and of the world. Realizing that classical physics fails catastrophically when things are atom-sized or smaller, he remodeled the atom so electrons occupied ‘allowed’ orbits around the nucleus while all other orbits were forbidden. In doing so he founded quantum mechanics.
Later, as a leading architect of the Copenhagen interpretation of quantum mechanics, he helped to reshape our understanding of how nature operates at the atomic scale.
Beginnings
Niels Henrik David Bohr was born on October 7, 1885 in Denmark’s capital city, Copenhagen. He was the second of three children in a prosperous, upper-class family.
His father was Christian Bohr, a brilliant physiology professor who would later be nominated twice for a Nobel Prize. His mother was Ellen Adler, daughter of a wealthy Danish politician.
His father had been raised in a Lutheran family and his mother in a Jewish family. Niels was baptized as a Lutheran at the age of six to please one of his grandmothers. Neither his father nor his mother practiced their religions.
Niels’ parents were deeply passionate about their children’s education. Niels was taught at home until he started formal schooling aged 7 at the Gammelholm Grammar School. The school was both an elementary and high school. It had strict discipline and expected its students to work hard.
His father brought home a variety of fellow professors from the University of Copenhagen and the Bohr children were allowed to listen to the conversations, which were wide-ranging, discussing science, philosophy, and the arts.
Physical and Intellectual Strength
Niels was good at most school subjects, but was rather weak in his own native language, Danish. While he loved talking, he had a thorough dislike of writing essays. Naturally talented in mathematics, he became increasingly drawn to the sciences.
Niels was good at most school subjects, but was rather weak in his own native language, Danish. While he loved talking, he had a thorough dislike of writing essays. Naturally talented in mathematics, he became increasingly drawn to the sciences.
Physics especially interested Niels and by the time he was a teenager he was correcting the mistakes in his schools’ textbooks. In addition to his intellectual vigor, he was also unusually strong physically. He didn’t just correct textbooks; he would also ‘correct’ other students, getting into fights at school, which he usually won.
Although he would eventually become one of the world’s greatest theoretical physicists, he was talented practically with his hands. He and his younger brother spent hours making things in their father’s workshop.
His father saw Niels had the potential to become an outstanding scientist. However, neither of Niels’ parents wanted their son to grow up with narrow interests. They ensured he was well-educated culturally and in sports.
His father was particularly enthusiastic about the works of the German author Goethe and would regularly recite large tracts of Faust to his children. His father was also loved soccer and encouraged his sons to play at school and university. Niels became a goalkeeper, while his younger brother Harald went on to play for Denmark on the international stage, winning an Olympic silver medal.

Left: Christian Bohr. Right: (from left to right): Jenny, Harald, Ellen, and Niels. All three of the Bohr children became graduates of the University of Copenhagen. Jenny became a teacher of history and Danish. Harald became an illustrious mathematician.
B.S. Degree and Some Remarkable Research Work
In 1903, aged 17, Niels graduated from high school. Later that year he began his studies at the University of Copenhagen. He studied astronomy, chemistry, mathematics, and majored in physics.
In 1903, aged 17, Niels graduated from high school. Later that year he began his studies at the University of Copenhagen. He studied astronomy, chemistry, mathematics, and majored in physics.
In February 1905, while he was working towards his degree, The Royal Danish Academy of Sciences announced a gold medal would be awarded for the best research paper on methods for measuring the surface tension of liquids. This was a prize intended for experienced scientists, not undergraduates. Niels was aware of his own growing strength in physics and he was ambitious; he decided he would enter the competition.
He was fortunate in having a professor for a father. His father allowed him space in his physiology laboratory to do experiments. For months Niels worked alone and obsessively during the night, making his own equipment and using it to form water jets and make measurements.
His father, recognizing his son’s growing obsession with the experiments, ordered him to stop and write up his research. Niels retreated to his wealthy maternal grandparents’ country estate to do this. The paper he submitted at the end of October 1906 was sufficiently brilliant to win him a gold medal – a remarkable achievement for an undergraduate. He shared the prize with Peder Pedersen, 11 years his senior, who would soon become a professor of electrical engineering.
Niels Bohr graduated with a B.S. degree in 1907.
M.S. and Ph.D.
In the years 1907 – 1911, Bohr completed his M.S. and Ph.D. degrees in physics. In both cases he turned his attention to the electron theory of metals. His Ph.D., awarded in April 1911, was a purely theoretical work.
In the years 1907 – 1911, Bohr completed his M.S. and Ph.D. degrees in physics. In both cases he turned his attention to the electron theory of metals. His Ph.D., awarded in April 1911, was a purely theoretical work.
Physics Explodes
Bohr was entering physics at a particularly exciting time.
Bohr was entering physics at a particularly exciting time.
- In 1897, when Bohr was 12, J. J. Thomsondiscovered the electron.
- In 1898 Ernest Rutherford discovered alpha and beta particles emitted by uranium.
- In 1905, when Bohr was beginning his gold medal winning research, Albert Einsteinunleashed a barrage of new ideas in his miracle year, writing four world-changing papers on: Brownian motion, the equivalence of mass and energy, the photoelectric effect, and special relativity.
- In 1909 Ernest Rutherford discovered the atomic nucleus.
A Bad Start with J. J. Thomson
Bohr was awarded funding for a year’s postdoctoral work overseas and had the good fortune to be one of the chosen few accepted to work in J. J. Thomson’s Cavendish Laboratory at the University of Cambridge in England. He arrived in October 1911.
Bohr, then 26 years old, made a bad start with Thomson. With an outrageous lack of subtlety, the first thing the young physicist said to the great man was ‘this is wrong’ and pointed to a page of a book authored by Thomson. Perhaps this unfortunate first meeting had something to do with it, but Bohr found it hard to get to grips with the Cavendish Lab’s work. The research did not appeal to him.
Ernest Rutherford – Things are Looking Up
By the end of 1911, Bohr had met another great physicist, Ernest Rutherford, whose laboratory was at the University of Manchester. He asked Rutherford if he could transfer there to work with him. Rutherford said yes, providing Bohr got Thomson’s approval first.
And so, in March 1912, Bohr caught the train to Manchester to work with the man who would both inspire him and become one of his greatest friends.
Rutherford had won the 1908 Chemistry Nobel Prize for his work in radioactivity. In 1909 he had discovered the atomic nucleus. However, despite Rutherford’s high reputation, his claim that the atom was made of a tiny, very dense, positively charged nucleus surrounded by negatively charged electrons had met with a lukewarm response from other physicists.
Bohr became increasingly interested in Rutherford’s model of the atom, particularly the behavior of its electrons, the subject of his Ph.D. thesis.
He and Rutherford became the greatest of friends and in years to come they and their wives would spend happy vacations together. 25 years after they first worked together, Bohr would stand with Rutherford’s family at the great man’s funeral.
Big Ideas
Bohr returned to Denmark in the fall of 1912 with two foremost ideas:
Firstly, he wanted to understand the behavior of electrons in the atom.
Secondly, he resolved to model his own behavior as a physicist on Rutherford’s. Bohr found Rutherford’s boundless energy, enthusiasm, and knowledge inspiring. Also he had found the intellectually exhilarating atmosphere of the Rutherford group highly agreeable. He hoped one day to build an equally outstanding research group in Copenhagen.
Niels Bohr’s Contributions to Science
A New Way of Thinking about Atoms
Bohr secured lecturing work on his return to the University of Copenhagen.
Meanwhile, his theoretical physics research focused on understanding the electron’s place in the atom.
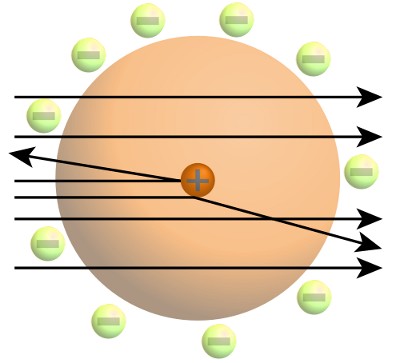
Rutherford’s work had revealed atoms were made up of a tiny dense positively charged nucleus. The great majority of an atom’s volume was empty space patrolled in some way by negatively charged electrons.
Bohr knew Rutherford’s picture of the atom disagreed with the laws of classical physics. These said that negatively charged electrons must radiate energy and be pulled into the positively charged nucleus. Even when he wrote his Ph.D. thesis, Bohr stated that it was impossible for classical physics to explain behavior at the atomic scale.
Now he looked to the new quantum physics of Max Planck and Albert Einstein for a solution to the apparently impossible behavior of electrons. In fact, he started on this track in Manchester in 1912.
Quantum physics had established that when an object radiates heat or light waves, the emission comes not in a continuous stream, but rather in distinct packets of wave energy.
Einstein called these distinct packets photons. Like all waves, photons have a speed, frequency and a wavelength.
Planck deduced that the amount of energy carried by a photon could be found by multiplying just two numbers. These were the light’s frequency and a number we now call the Planck constant. His equation said E = hf, where E is energy, h is the Planck constant, and f is frequency.
Clearly a photon could only carry an amount of energy that was a multiple of one number – Planck’s constant. All other energies were forbidden. This was the essence of quantum theory – light was allowed to have certain amounts of energy, but was forbidden from having others.
No matter how hard Bohr worked, no matter how much literature he read, and no matter how much he discussed the problem with colleagues, he could not find a way of bringing quantum theory – in other words, allowed states and forbidden states – into the electron’s behavior in the atom.
Then, in February 1913, came the breakthrough. He heard about the Balmer Series and the Balmer Formula.

Part of the Balmer Series. These colors of light are emitted by hydrogen at high temperatures. The wavelength and frequency of the different colors of light can be fitted to a formula – the Balmer Formula.
In 1885 the Swiss mathematician Johann Balmer had stumbled upon a mathematical formula that predicted the wavelengths – and hence colors – of light emitted by hot hydrogen. There was no theoretical basis for the formula. It just worked!
Now Bohr felt he was really on to something. He took Balmer’s formula and used the new quantum theory to show why it worked. In doing so, he became the father of quantum mechanics – the physics of atom-scale objects.
Bohr proposed that the relation E = hf lay at the heart of explaining the behavior of electrons. Just like light energy came in distinct packets of energy, so did electrons. Only certain values of electron energy were allowed, and they could be calculated for hydrogen using Balmer’s formula.
Into Balmer’s formula Bohr substituted Planck’s formula and some other important numbers including the electron’s mass, and its charge.
The mathematical result, in simple terms, was an atom that can be pictured as a tiny solar system. Just as planets orbit the sun, electrons orbit the atomic nucleus in fixed orbits. The farther an electron is from the nucleus, the higher its energy. Unlike planets, more than one electron can share an orbit around the nucleus.
Bohr Sheds New Light on an old Problem
Scientists had long wondered precisely how matter could absorb and emit light. Bohr’s new model of the atom offered the explanation.
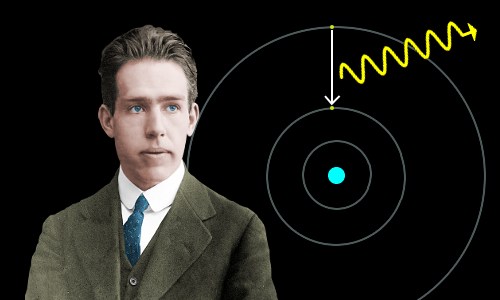
Bohr said electrons are restricted to particular circular orbits, but can jump from a lower energy orbit to a higher energy orbit by absorbing light. They can also do the opposite and fall from a higher energy orbit to a lower energy orbit by emitting light – as shown in the image below.
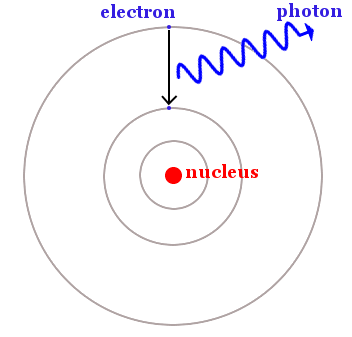
An electron falls from a higher energy orbit to a lower energy orbit. Energy is released as a photon of light. The difference in energy between the orbits is the same as the energy of the photon, which can be calculated using Planck’s equation, E = hf.
In classical physics, electrons could have any energy. In the new quantum physics electrons were confined to defined orbits of fixed energy. Other electron energies were forbidden.
When an electron absorbed energy it made a quantum leap, disappearing from one orbit and appearing in a higher one across a forbidden energy zone. When an electron lost energy it disappeared from a higher energy orbit and appeared in a lower energy orbit separated by a forbidden zone.
Quantum theory ‘explained’ why electrons do not radiate away their energy as they fall into the nucleus, because this process is forbidden: the nucleus is not an allowed energy orbit for an electron.
Quantum theory also explained the spectra of atoms, showing the intense colors in spectra were related to the energies of electron orbits in atoms.

The emission spectrum of helium. The intense lines reveal the energies of the allowed electron orbits in helium.
The Founding of Quantum Chemistry
Bohr showed that the chemical properties of the elements result mainly from the behavior of electrons occupying the highest stable orbit – these are called the valence electrons. In doing so, he explained much of the periodic table’s structure and founded a new scientific discipline – quantum chemistry.
Bohr Pushes our Understanding of the Atom Forward by a Quantum Leap
In 1912 many scientists had not even taken on board Rutherford’s model. In 1913 Bohr combined mathematics with his powerful physical intuition to establish that electrons orbit the nucleus in defined paths. The Rutherford-Bohr model of the atom is still taught in high schools.
Bohr opened the atom to quantum theory: it was an object that changed its state in steps rather than smoothly.
However, his theory only worked well for atoms with one electron – in other words hydrogen or ionized helium. Nevertheless Bohr’s was the crucial step – he opened the door. Soon the quantum world was knee-deep in industrious physicists eagerly exploring its bizarre nooks and crannies.
 “The whole field of work has indeed from a very lonely state suddenly got into a desperately crowded one where almost everybody seems hard at work.”
“The whole field of work has indeed from a very lonely state suddenly got into a desperately crowded one where almost everybody seems hard at work.”
NIELS BOHR
Letter to Ernest Rutherford, September 6, 1916
In the 1920s Werner Heisenberg and Erwin Schrödinger provided a much improved quantum view of the electron’s place in the atom.
Bohr in Demand
Bohr published three famous quantum papers in 1913. In doing so, his reputation as a physicist enjoyed its very own quantum leap. He gave seminars at the University of Göttingen in Germany – the center of the mathematical universe – and accepted an offer from Ernest Rutherford to return to Manchester in a senior academic research role.
The outbreak of World War 1 complicated matters, but Bohr worked from 1914 – 1916 in Manchester.
He then returned to Copenhagen to be the university’s first chair of theoretical physics. He raised money to establish a theoretical physics research institute as part of the university, modeled on Rutherford’s research group. Werner Heisenberg and several other architects of the new quantum mechanics developed their ideas in Copenhagen under Bohr’s leadership.
Today the Niels Bohr Institute continues to operate at the forefront of the physical sciences.
Nobel Prize
Bohr was awarded the 1922 Nobel Prize in Physics for the work he did in 1913.
The Copenhagen Interpretation of Quantum Mechanics
As quantum mechanics developed, different views of how it should be interpreted emerged. There was a great intellectual dispute (always good-natured rather than bitter) between Einstein and Bohr about whether, to paraphrase Einstein, God plays dice with the universe. Einstein said no, Bohr said yes.

Bohr and Einstein enjoyed each other’s company and agreed about many things, but they could not agree about the quantum world.
Today the great majority of physicists accept Bohr’s proposition that God does play dice with the universe.
At the atomic-scale the world can look outlandish to anyone hoping to interpret it according to the laws of ‘common sense’ or indeed the rules of classical physics. It is a world where something we normally think of as a wave, such as light for example, can behave like a particle. Or something we normally think of as a particle, such as an electron, can behave like a wave. A single neutron might appear to be in two places simultaneously, as much as a few centimeters apart. It is a world where a cat can be thought of as both alive and dead (or in some intermediate state) at the same time.
Three of the giants of twentieth century physics – Niels Bohr, Werner Heisenberg and Wolfgang Pauli – were the key players in developing what came to be known as the Copenhagen interpretation of quantum mechanics. Pauli had to mediate between Bohr and Heisenberg (who was working in Bohr’s institute) because at times they disagreed so fiercely about how the quantum world should be interpreted that they could barely speak to one another.
From this scorching intellectual crucible, four of the most important ideas of what came to be known as the Copenhagen interpretation of quantum theory emerged. These were:
- Bohr’s principle of complementarity/Heisenberg’s uncertainty principle
- Wave-particle duality
- Interpretation of wave-functions using probabilities
- The correspondence principle – the merging of quantum mechanics into classical mechanics at larger quantum numbers
Whatever the philosophical implications of the Copenhagen interpretation might be – and there are plenty – for example that reality does not exist unless you look for it – what we know for sure is that the methods of quantum mechanics work in practice.
Quantum mechanics is the most powerful tool we have ever had for figuring out how the universe works at the atomic scale.
The Compound Nucleus
With James Chadwick’s discovery of the neutron in Rutherford’s laboratory in 1932, Bohr turned his attention to the atomic nucleus.
Nuclear reactions fascinated him, particularly reactions in which atomic nuclei were bombarded with neutrons to form new, radioactive nuclei – neutron capture reactions.
Dissatisfied with the attempts of other physicists to explain neutron capture, Bohr formulated the compound nucleus theory in 1934 and 1935, publishing it in 1936. His idea was that when a neutron enters a nucleus, it repeatedly collides with a large number of existing neutrons and protons, not just one of them. The result is a semi-stable compound nucleus. This nucleus is in a high-energy state as a result of the collisions and loses this energy in different ways, such as losing a neutron or emitting gamma rays.
Bohr’s theory held center-stage for the following two decades, until in the 1950s his son Aage played a key role in formulating an improved model of the nucleus and nuclear reactions.
The Nucleus as a Drop of Liquid
In 1939 Niels Bohr and John Archibald Wheeler cooperated to produce the liquid-drop model. This model pictured the nucleus as a rotating drop of incompressible liquid held together by surface tension.
A drop of liquid can be deformed from its basic spherical shape and a large drop of liquid can fall apart into two new drops. Similarly a large atomic nucleus, like uranium, could fall apart to form two new atomic nuclei – this is nuclear fission, the energy source behind both the uranium atom bomb and the uranium power plant.
The tiny atomic nucleus was modeled as a drop of liquid held together by surface tension. Just like a liquid, the shape of the drop was spherical, but could be deformed from this shape.
The liquid drop model had its greatest successes in explaining the properties of heavy nuclei, such as uranium.
The Manhattan Project
In April 1940 Denmark was invaded by the armed forces of Nazi Germany. Bohr’s mother was Jewish, which meant he could face problems. At first, thankfully, there were none.
In September 1943 the Nazis decided to deport Denmark’s Jews to concentration camps. Bohr received a tip-off that the Nazis intended to arrest him. The Bohr family fled in fishing boats across the short stretch of water separating Denmark from Sweden. Sweden was officially neutral and had not been invaded by the Nazis. Nearly all of Denmark’s 7000 Jews fled over the sea to Sweden in 1943.
In October 1943, one week apart, Niels and his son Aage flew from Sweden over Nazi-occupied Norway to the United Kingdom. They flew in the bomb bays of British warplanes that came to Sweden to collect them. Margrethe Bohr remained in Sweden until the war ended.

At the age of 58, Niels Bohr was flown from Sweden high over Nazi-occupied Norway and the North Sea to Scotland in a de Havilland Mosquito. During the flight he lay in the place the aircraft’s bombs would normally be placed. His oxygen supply failed during the flight and he lost consciousness. The pilot, after losing communication with Bohr, guessed what had happened and brought the aircraft down to a lower elevation. Bohr recovered consciousness by the time they landed.
Once safely in the UK, father and son began scientific research for the British Government, working in the atomic bomb project headed by James Chadwick.
In 1944 father and son became involved in the Manhattan Project, spending significant amounts of time in the United States as well as London. To keep their presence in America secret, they traveled under the names “Nicholas Baker” and “James Baker.”
Some Personal Details and the End
In 1912, Bohr married Margrethe Nørlund in Copenhagen. They had six sons, one of whom, Aage Bohr would emulate his father by winning a Nobel Prize in Physics.
Niels Bohr died aged 77 of sudden heart failure in his home in Copenhagen on November 18, 1962. His ashes were buried in Copenhagen’s Assistens Cemetery near the graves of his parents and his brother Harald. Margrethe’s ashes were also buried there when she died.
No comments:
Post a Comment