André Marie Ampère
Lived 1775 – 1836.
André-Marie Ampère made the revolutionary discovery that a wire carrying electric current can attract or repel another wire next to it that’s also carrying electric current. The attraction is magnetic, but no magnets are necessary for the effect to be seen. He went on to formulate Ampere’s Law of electromagnetism and produced the best definition of electric current of his time.
Ampère also proposed the existence of a particle we now recognize as the electron, discovered the chemical element fluorine, and grouped elements by their properties over half a century before Dmitri Mendeleev produced his periodic table.
The SI unit of electric current, the ampere, is named in his honor.
Beginnings
André-Marie Ampère was born into a well-to-do family in the city of Lyon, France, on January 20, 1775. His father was Jean-Jacques Ampère, a businessman; his mother was Jeanne Antoinette Desutières-Sarcey, the orphaned daughter of a silk-merchant. André-Marie’s parents already had a daughter, Antoinette, born two years before André-Marie.
It was an intellectually exciting period in French history; Antoine Lavoisier was revolutionizing chemistry; and Voltaire and Jean-Jacques Rousseau, the leaders of the French Enlightenment, were urging that society should be founded on science, logic, and reason rather than the religious teachings of the Catholic Church.
When André-Marie was five years old, his family moved to a country estate near the village of Poleymieux about six miles (10 km) from Lyon. His father had grown so wealthy that he no longer needed to spend much time in the city. A second daughter Josephine was born when André-Marie was eight.
An Unusual Education
The education André-Marie received was rather unusual. His father was a great admirer of Jean-Jacques Rousseau, one of the leaders of the French Enlightenment. He decided to follow Rousseau’s approach for André-Marie’s education. This meant no formal lessons.
The education André-Marie received was rather unusual. His father was a great admirer of Jean-Jacques Rousseau, one of the leaders of the French Enlightenment. He decided to follow Rousseau’s approach for André-Marie’s education. This meant no formal lessons.
André-Marie could do as he pleased, learning about anything he felt like. He was also allowed to read anything he wanted to from his father’s large library. A recipe for disaster, you may think? In fact, it worked! And it worked exceptionally well. André-Marie developed an insatiable thirst for knowledge, going as far as learning entire pages of an encyclopedia by heart.
Although a child of the French Enlightenment, André-Marie did not reject the church, and he remained a practicing Catholic throughout his life.
 “My father… never required me to study anything, but he knew how to inspire in me a great desire for knowledge. Before learning to read, my greatest pleasure was to listen to passages from Buffon’s natural history. I constantly requested him to read me the history of animals and birds…”
“My father… never required me to study anything, but he knew how to inspire in me a great desire for knowledge. Before learning to read, my greatest pleasure was to listen to passages from Buffon’s natural history. I constantly requested him to read me the history of animals and birds…”
ANDRÉ-MARIE AMPÈRE, 1775 – 1836
Memoirs, switched from third person to first person.
Mathematics
Aged 13, André-Marie began a serious study of mathematics using books in his father’s library. He submitted a paper about conic sections to the Academy of Lyon, but it was rejected.
Aged 13, André-Marie began a serious study of mathematics using books in his father’s library. He submitted a paper about conic sections to the Academy of Lyon, but it was rejected.
The rejection spurred him into working harder than ever. His father bought him specialist books to help him improve. He also took his son into Lyon, where Abbot Daburon gave him lessons in calculus – the first formal lessons André-Marie ever had.
Physics
Having taken his son for formal mathematics lessons, his father also took him to Lyon’s college to attend some physics lectures, which resulted in André-Marie beginning to read physics books as well as mathematics books.
Having taken his son for formal mathematics lessons, his father also took him to Lyon’s college to attend some physics lectures, which resulted in André-Marie beginning to read physics books as well as mathematics books.
Revolution Followed by Tragedies
Life so far had been peaceful and enjoyable for André-Marie, but a period of tragedy was beginning to unfold.
Life so far had been peaceful and enjoyable for André-Marie, but a period of tragedy was beginning to unfold.
In 1789, when André-Marie was 14, the French Revolution began.
In 1791, while André-Marie continued his home-studies on their country estate, the revolutionaries gave his father the legal role of Justice of the Peace.
In 1792, André-Marie’s older sister Antoinette died.
In 1793, the Jacobin faction of the revolution guillotined his father. (The great chemist Antoine Lavoisier was guillotined by revolutionaries in 1794.)
Mercifully, André-Marie, studying mathematics and science on the family estate, survived the revolution’s reign of terror. He was devastated by his father’s death and abandoned his studies for a year.
Ampere’s Lifetime in Context
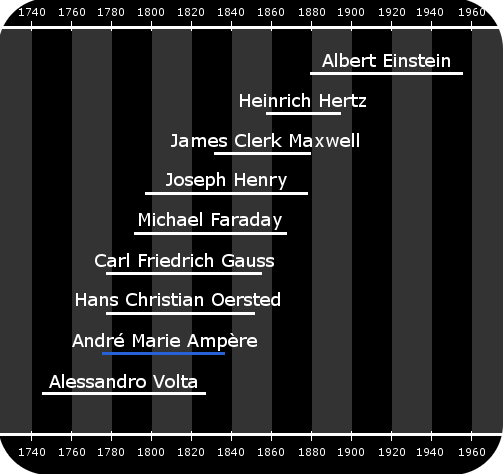
Ampere’s lifetime and the lifetimes of related scientists and mathematicians.
Becoming a Mathematician and Scientist
In late 1797, aged 22, André-Marie Ampère opened up shop as a private mathematics tutor in Lyon. He proved to be an excellent tutor, and soon students were flocking to him for help.
His tutoring work came to the attention of Lyon’s intellectuals, who were impressed by Ampère’s knowledge and his enthusiasm.
In 1802, he became a school teacher in the town of Bourg 40 miles (60 km) from Lyon. A year later he returned to Lyon to work in another teaching position.
In 1804, he moved to the French capital, Paris, tutoring university level classes at the École Polytechnique. His work impressed other mathematicians so much that he was promoted to full professor of mathematics in 1809, despite having no formal qualifications.
André-Marie Ampère’s Contributions to Science
Electromagnetism and Electrodynamics
In 1800, while Ampère worked as a private tutor in Lyon, Alessandro Volta had invented the electric battery. One result of this was that for the first time ever, scientists could produce a steady electric current.
In April 1820, Hans Christian Oersted discovered that a flow of electric current in a wire could deflect a nearby magnetic compass needle. Oersted had discovered a link between electricity and magnetism – electromagnetism.
In September 1820, François Arago demonstrated Oersted’s electromagnetic effect to France’s scientific elite at the French Academy in Paris. Ampère was present, having been elected to the Academy in 1814.
Ampère was fascinated by Oersted’s discovery and decided he would try to understand why electric current produced a magnetic effect.
 “Ever since I first heard of Oersted’s great discovery… of the action of electric current on a magnetized needle, I have thought about it constantly. All my time has been dedicated to writing a great theory about these phenomena… and attempting the experiments indicated by this theory, all of which succeeded.”
“Ever since I first heard of Oersted’s great discovery… of the action of electric current on a magnetized needle, I have thought about it constantly. All my time has been dedicated to writing a great theory about these phenomena… and attempting the experiments indicated by this theory, all of which succeeded.”
ANDRÉ-MARIE AMPÈRE, 1775 – 1836
Communication to his son, Jean-Jacques, 1820
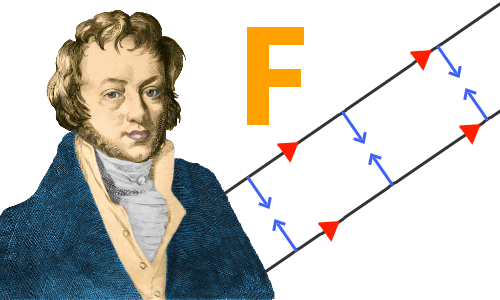
Ampère began by repeating Oersted’s work, and before the end of September 1820, had made a discovery of his own: he found that if electric current flows in the same direction in two nearby parallel wires, the wires attract one another; if electric currents flow in opposite directions the wires repel one another.
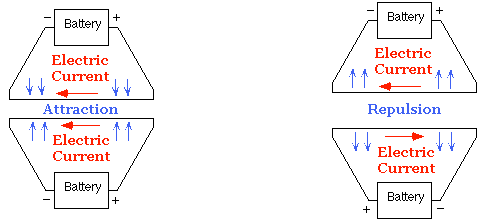
Ampère found that parallel wires with currents flowing in the same direction attract each other. Currents in opposite directions repel each other.
Ampère had discovered something amazing: he had produced magnetic attraction and repulsion in the complete absence of any magnets. All of the magnetism was generated electrically. He called this new field electrodynamics. (Today electrodynamics and electromagnetism are regarded as the same field.)
Ampère’s Law
Ampère then brilliantly found an equation connecting the size of a magnetic field to the electric current that produces it. This equation, known as Ampère’s circuital law, is highly mathematical, requiring university level mathematics to use and understand. It is shown below in differential form relating the magnetic field (B) to the current density (J).
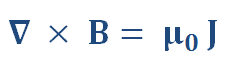
This equation applies to situations where the electric current is constant. Over 40 years later, James Clerk Maxwell modified this equation so it would also apply to situations in which the current is not constant. In this form it became one of his four famous equations establishing that light is an electromagnetic wave.
 “The experimental investigation by which Ampère established the law of the mechanical action between electric currents is one of the most brilliant achievements in science. The whole theory and experiment, seems as if it had leaped, full grown and full armed, from the brain of the ‘Newton of Electricity’. It is perfect in form, and unassailable in accuracy, and it is summed up in a formula from which all the phenomena may be deduced, and which must always remain the cardinal formula of electrodynamics.”
“The experimental investigation by which Ampère established the law of the mechanical action between electric currents is one of the most brilliant achievements in science. The whole theory and experiment, seems as if it had leaped, full grown and full armed, from the brain of the ‘Newton of Electricity’. It is perfect in form, and unassailable in accuracy, and it is summed up in a formula from which all the phenomena may be deduced, and which must always remain the cardinal formula of electrodynamics.”
JAMES CLERK MAXWELL, 1831 – 1879
Electricity and Magnetism, Vol. 2, Chapter 3
The Electron
To explain the relationship between electricity and magnetism, Ampère proposed the existence of a new particle responsible for both of these phenomena – the electrodynamic molecule, a microscopic charged particle we can think of as a prototype of the electron. Ampère correctly believed that huge numbers of these electrodynamic molecules were moving in electric conductors, causing electric and magnetic phenomena.
Discovery of Fluorine
Ampère did not restrict his interests to mathematics and physics; they were wide ranging and included philosophy and astronomy. He was particularly interested in chemistry. In fact, preceding his work in electromagnetism, he made significant contributions to chemistry.
Ampère discovered and named the element fluorine. In 1810, he proposed that the compound we now call hydrogen fluoride consisted of hydrogen and a new element: the new element had similar properties to chlorine he said. He and Humphry Davy, who was British, entered into correspondence (even though France and Britain were at war). Ampère proposed that fluorine could be isolated by electrolysis, which Davy had previously used to discover elements such as sodium and potassium.
It was only in 1886 that French chemist Henri Moissan finally isolated fluorine. He achieved this using electrolysis, the method Ampère had recommended.
 “During the period I was engaged in these investigations, I received two letters from M. Ampère of Paris, containing many ingenious and original arguments in favour of the analogy between the muriatic [chlorine] and fluoric [fluorine] compounds.”
“During the period I was engaged in these investigations, I received two letters from M. Ampère of Paris, containing many ingenious and original arguments in favour of the analogy between the muriatic [chlorine] and fluoric [fluorine] compounds.”
SIR HUMPHRY DAVY, 1778 – 1829
Philosophical Magazine, Volume 42., p408, 1813
Organizing the Chemical Elements
In 1816, 53 years before Dmitri Mendeleev published his periodic table, Ampère proposed that the chemical elements – 48 were known at that time – should be grouped according to their properties. He made a number of mistakes but successfully grouped:
- the alkali metals: sodium and potassium
- the alkali earth metals: magnesium, calcium, strontium, and barium
- the halogens: chlorine, fluorine, and iodine
He was also moving in the right direction by identifying similarities in:
- the noble metals: rhodium, palladium, iridium, platinum, and gold (unfortunately, Ampère excluded silver from this group, grouping it instead with mercury, lead, and bismuth)
- first series transition elements: iron, cobalt, nickel, copper, (although uranium was incorrectly included)
- transition elements: niobium, molybdenum, chromium, and tungsten
Ampère did not name the groups as they are named above, such as the noble metals and transition elements – these are modern names.
Mendeleev had an advantage over Ampère in that 65 elements were known to him, allowing him to see patterns more easily. Importantly, Mendeleev also paid attention to atomic weights, while Ampère did not. To be fair to Ampère, we need to remember that J. J. Berzelius published the first reasonably accurate list of atomic weights in 1828, 12 years after Ampère’s elements work.
 “…it seemed to me that one should make an effort to banish artificial classifications from chemistry and begin to assign to each element the place it must occupy in the natural order by comparing it in succession to others…”
“…it seemed to me that one should make an effort to banish artificial classifications from chemistry and begin to assign to each element the place it must occupy in the natural order by comparing it in succession to others…”
ANDRÉ-MARIE AMPÈRE, 1775 – 1836
Annales de Chimie et de Physique, Vol 2.
The Ampere
The SI unit for electric current is the ampere or amp (symbol A), named in Ampère’s honor. It was Ampère who first defined electric current as a ‘circulation of electric fluid in a closed circuit.’
Some Personal Details and the End
In 1799, aged 24, Ampère married 25-year-old Catherine-Antoinette Carron, who was usually called Julie. A year later, their son Jean-Jacques was born – he was named in memory of Ampère’s beloved father. Tragedy struck Ampère when, after less than four years of marriage, Julie died in 1803 of abdominal cancer.
Ampère got married again in 1806, to Jeanne-Françoise Potot. The couple quickly realized their marriage had been a mistake. Their daughter Albine was born in 1807, and the couple legally separated in 1808. Albine came to live with her father and his younger sister Josephine.
In 1824, Ampère was appointed to the Chair of Experimental Physics at the Collège de France in Paris, which he occupied for the rest of his life.
Ampère’s son, Jean-Jacques, became a notable professor of linguistics and a member of the French Academy. He and his father were known to have rather explosive arguments with one another, both having quick tempers.
At the age of 61, Ampère caught pneumonia. He died in the French Mediterranean city of Marseilles on June 10, 1836.
He was buried in Marseilles, but his remains were later moved to the Montmartre Cemetery in Paris. Many other illustrious people are buried in Montmartre Cemetery, including the composers Hector Berlioz and Jacques Offenbach; the artist Edgar Degas; the author Émile Zola; the physicist Léon Foucault; the mathematician Stanislaw Ulam; and Ampère’s son, Jean-Jacques, who is buried next to his father.
Excellent blog. Well done.
ReplyDeletewell done:)
ReplyDelete